Underwater videography has become a staple of observational studies in both tropical and temperate environments, where the technique offers a robust, non-invasive, and affordable means of monitoring marine species in situ (Mallet & Pelletier 2014). Initially pioneered for applications in the abyssal zone (Priede et al. 1994), benthic BRUVs (see https://benthic-bruvs-field-manual.github.io/) have been extensively used in shallow, inshore environments (e.g. McLean et al. 2011, Langlois et al. 2012, Zintzen et al. 2012, Oh et al. 2017, Juhel et al. 2018).
However, a growing international commitment to expand the world’s marine protected area coverage in recent years (Pala 2013) has motivated efforts to adapt BRUVs to pelagic, open ocean habitats away from coasts (Bouchet & Meeuwig 2015) (Table 6.1). Multiple research groups and organisations have concurrently developed several pelagic BRUV designs (Figure 6.1), most of which share similar elements, namely (i) one (monocular) or a pair (stereo) of cameras in appropriate underwater housings, (ii) a base frame on which the camera(s) is/are mounted, (iii) an attractant, usually olfactory in the form of bait, (iv) a synchronisation device (e.g. diode, clapperboard) and (iv) a suspension system (consisting of weights, ropes, and floats).
Pelagic BRUVs retain all the qualities that have made camera-based sampling a flexible and effective approach to non-destructive marine monitoring, as:
- They are suitable in areas where fishing or other extractive activities are prohibited.
- They are straightforward and relatively quick to operate.
- They have little direct impact on wildlife and ecosystems, other than through bait use.
- They present a safety advantage over diver-based methods and overcome some of their limitations and biases (e.g. depth and time constraints, avoidance behaviour in fishes).
- They produce accurate body length measurements when configured in stereo.
- They yield a permanent archive of high-definition footage.
- They generate quantitative data, while also documenting behaviour.
- They are viable in a range of depths, underwater terrains and ocean conditions.
Importantly, the use of one or more attractants substantially increases the likelihood that nearby animals enter the field of view of the cameras for digital capture (Rees et al. 2015). Extensive collective experience in the deployment of pelagic BRUVs across a range of habitats, climates, and conditions indicates that the instruments are capable of detecting a large suite of taxonomic groups (including many of interest to fisheries), from teleost fishes to elasmobranchs, marine mammals, molluscs, crustaceans, and reptiles (Figure 6.2).
In spite of their performance, pelagic BRUVs suffer from a number of limitations, many of which apply equally to demersal videography, including:
- Footage quality is affected by high turbidity and low visibility.
- Correct identification of some species can be difficult for small, shy or morphologically similar species and individuals.
- Bait dispersal is a complex, dynamic process likely to fluctuate spatio-temporally. Quantifying the size of the effective area being sampled and its variation remains an unresolved challenge.
- Bait elicits diverse animal behavioural responses whose strength, timing and duration often relate to many unknown parameters (e.g. olfactory performance, prey search strategy, human presence etc.).
- Numerous species may also respond to non-olfactory cues in ways that have seldom been quantified (but see Rees et al. 2015).
- The nature and magnitude of observation biases arising from the presence of conspecifics (and other species) are largely unknown (Dunlop et al. 2014, Coghlan et al. 2017).
- Counts of wildlife on BRUVs reflect measures of relative rather than absolute abundance and can be biased, e.g. by screen saturation (Lowry et al. 2011, Schobernd et al. 2013).
- Detection/attraction probabilities likely vary by time of day, habitat, bathome, and species.
- Zero-inflation is common and may undermine the statistical power needed to identify patterns and changes in pelagic communities (Santana-Garcon et al. 2014b).
- Benthic “species contamination” can occur wherever the ratio between suspension and seabed depths approaches one (e.g. pelagic BRUVs suspended at 10 m in a total of 15 m of water) (Letessier et al. 2013b), but see Clarke et al. (2019) for a comparison of benthic and pelagic assemblages and their overlap at different depths.
Further discussion of some of these caveats can be found in Bouchet & Meeuwig (2015), Santana-Garcon et al. (2014b) and Espinoza et al. (2014), among many others.
Table 6.1: Summary of studies using pelagic video systems in marine monitoring. Orientation refers to the angle of the camera(s), and can be either horizontal (forward-facing) or vertical (downward-facing). Deployments can be conducted with instruments either moored to the seafloor (‘anchored’), linked to a vessel via a coaxial cable or similar (‘tethered), or free drifting (as individual units or in a longline configuration). NSW: New South Wales. WA: Western Australia. Due to differences in local supply, it is difficult to identify a standardised type of baitfish. As a rule, small pelagic species with soft, oily flesh are usually recommended. For instance, sardines/pilchards (Sardinops sagax) have been a staple of BRUV research in Australia and New Zealand, as evidence suggests they result in consistent numbers of fish among samples (less variation), exhibit higher mean abundance among sites and are more persistent (i.e. longer time to depletion) (Dorman et al. 2012). MW = mid-water. P = pelagic. S = Stereo.
| Authors | Location | Stereo | Orientation | Method | Attractant type | Bait type | Instrument name |
|---|---|---|---|---|---|---|---|
| Heagney et al. (2007) | Lord Howe Island (NSW, Australia) |
🗶 | Horizontal | Anchored | Olfactory (dead bait) | Mixture of minced pilchards, bread and tuna oil (8:1:1), combined in matrix of vegetable meal (falafel) [100g] | MW BRUVs |
| Letessier et al. (2013) | Shark Bay (WA, Australia) |
✔ | Horizontal | Anchored | Olfactory (dead bait) | Pilchards, squid, and combination (slurry, 1:1) | MW camera rigs |
| Santana et al. (2014a) | Ningaloo Reef (WA, Australia) |
✔ | Horizontal | Anchored | Olfactory (dead bait) | Mullets (cut in halves) [1kg] | PS BRUVs |
| Santana et al. (2014b) | Coral Bay (WA, Australia) |
✔ | Horizontal | Anchored | Olfactory (dead bait) | Pilchards [800g] | PS BRUVs |
| Santana et al. (2014c) | Western Australia (several locations) | ✔ | Horizontal | Anchored | Olfactory (dead bait) | Crushed pilchards [800g] | PS BRUVs |
| Santana et al. (2014d) | Houtman Abrolhos Is. (WA, Australia) | ✔ | Horizontal | Anchored | Olfactory (dead bait) | Crushed pilchards [800g] | PS BRUVs |
| Schifiliti et al. (2014) | Ningaloo Reef (WA, Australia) |
✔ | Vertical | Tethered | Olfactory (dead bait) | N/A | RemORA |
| Bouchet & Meeuwig (2015) | Perth Canyon (WA, Australia) |
✔ | Horizontal | Drifting | Olfactory (dead bait) | Crushed pilchard heads, guts and tails [2-3kg] | PS BRUVs |
| Fukuba et al. (2015) | Mariana Trench (Western North Pacific) | 🗶 | Vertical | Drifting | Olfactory (live bait) | Live matured eels | Una-Cam |
| Rees et al. (2015) | Jervis Bay (NSW, Australia) |
🗶 | Horizontal | Anchored | Olfactory, visual, acoustic | Visual: Spearfishing ‘swivel flasher’. Acoustic: Playback recording of bait fish. Olfactory: Mixture of white bread and pilchards. |
MW RUVs |
| Scott et al. (2015) | Sydney Harbour (Australia) | 🗶 | Horizontal | Anchored | Olfactory (dead bait) | Mixture of minced pilchards, bread, andtuna oil, in an (8:1:1) [100g] | P BRUVs |
| Kempster et al. (2016) | Mossel Bay (South Africa) |
✔ | Vertical | Tethered | Olfactory (dead bait) | Sardines and fish heads [0.5kg] | RemORA |
| Vargas et al. (2016) | Australian east coast (several locations) | 🗶 | Horizontal | Drifting | Olfactory (dead bait) | Chopped pilchards and squid [500g] | Surf-BRUVs |
| Acuña-Marrero et al. (2018) | Galapagos Islands, Ecuador | ✔ | Horizontal | Anchored | Olfactory (dead bait) | Yellow-fin tuna [800g] | P BRUVs |
| Caselle et al. (2018) | Tristan da Cunha (British Overseas Territory) | ✔ | Horizontal | Drifting | Olfactory (dead bait) | Crushed fish [800g] | MW BRUVs |
| Ryan et al. (2018) | Mossel Bay (South Africa) |
✔ | Vertical | Tethered | Olfactory (dead bait) | Crushed sardines [0.5kg] | N/A |
| Clarke et al. (2019) | Gulf St Vincent (SA, Australia) | 🗶 | Horizontal | Anchored | Olfactory, visual | Visual: flasher. Olfactory: minced sardines [1kg] |
P BRUVs |
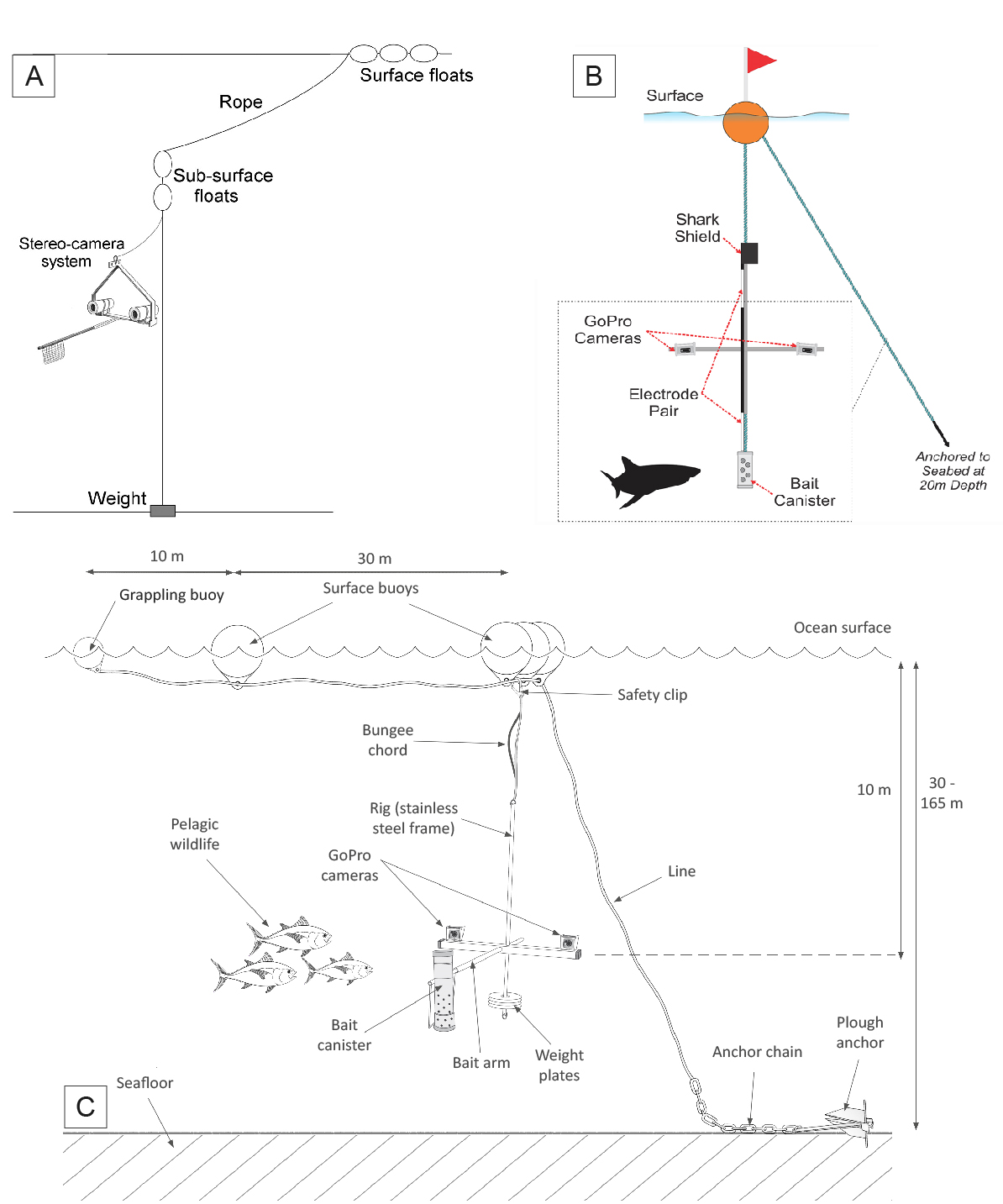
Figure 6.1: Examples of possible deployment configurations for pelagic BRUV sampling. Schematics extracted from or as used in (A) Santana-Garcon et al. (2014b), (B) Schifiliti et al. (2014) and Kempster et al. (2016), (C) Letessier et al. (2013b). Cameras can be either forward-facing (A, C) or downward-facing (B). The anchored design shown in C was adapted in Bouchet & Meeuwig (2015) to let BRUV units drift freely.

Figure 6.2: Example species observed on pelagic BRUVs. (A) Bryde’s whale Balaenoptera brydei, (B) Manta ray Manta birostris, (C) Dusky dolphin Lagenorhynchus obscurus, (D) Whale shark Rhincodon typus, (E) Dolphin fish Coryphaena hippurus, (F) Atlantic horse mackerel Trachurus trachurus, (G) Blue shark Prionace glauca, (H) Shortfin mako shark Isurus oxyrinchus, (I) Sea snake Hydrophiidae sp., (J) Green turtle Chelonia mydas, (K) Krill Euphausia sp., (L) Loggerhead turtle Caretta caretta, (M) Atlantic spotted dolphin Stenella frontalis, (N) Longfin yellowtail Seriola rivoliana, (O) Sub-Antarctic fur seal Arctocephalus tropicalis, (P) Yellowfin tuna Thunnus albacares, (Q) Pilot fish Naucrates ductor, (R) Blue marlin Makaira nigricans, and (S) Unicorn leatherjacket Aluterus monoceros.

